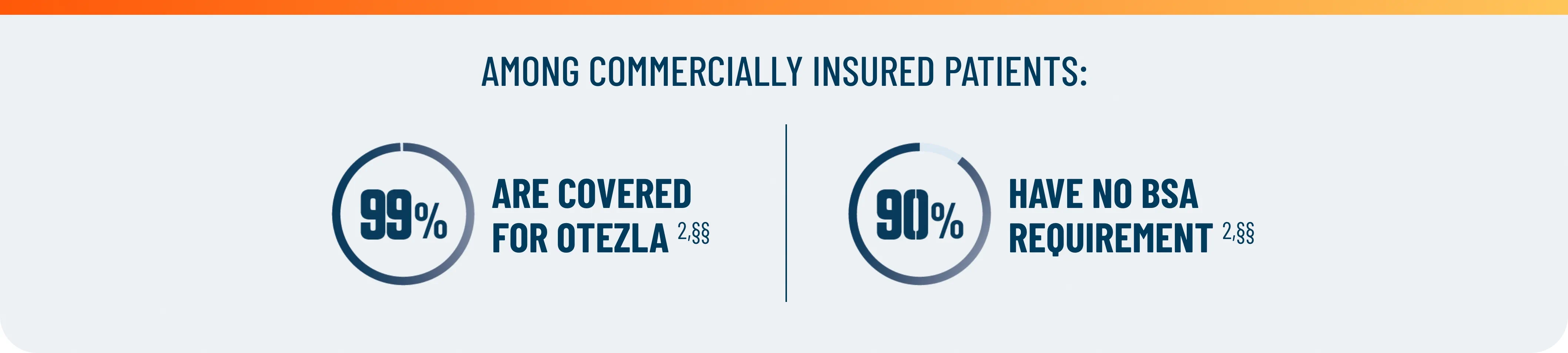
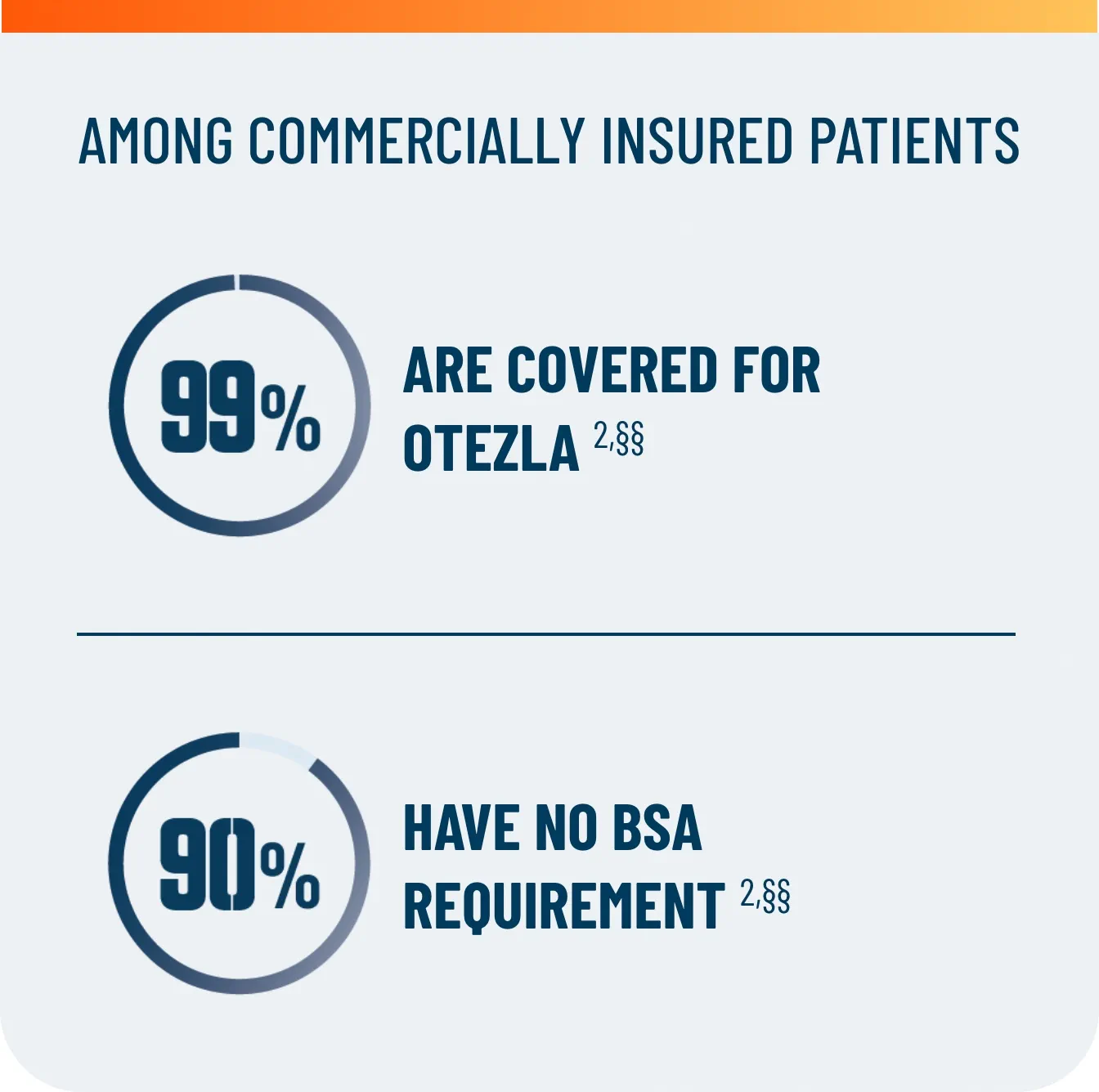
First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
OTEZLA:
4 INDICATIONSOtezla® (apremilast)/Otezla XR™ (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.Read more
OTEZLA:
4 INDICATIONS
- Otezla® (apremilast)/Otezla XR™ (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.
- Otezla is indicated for the treatment of pediatric patients 6 years of age and older and weighing at least 20 kg with moderate to severe plaque
psoriasis who are candidates for phototherapy or systemic therapy. - Otezla XR is indicated for the treatment of pediatric patients 6 years of age and older and weighing at least 50 kg with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
- Otezla is indicated for the treatment of adult patients and pediatric patients 6 years of age and older and weighing at least 20 kg with active psoriatic arthritis.
- Otezla XR is indicated for the treatment of adult patients and pediatric patients 6 years of age and older and weighing at least 50 kg with active psoriatic arthritis.
- Otezla/Otezla XR is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
- Clinical Response of Oral Ulcers
- Oral Ulcers: Pain Results
- Oral Ulcers: Complete Response
- Oral Ulcers: Maintenance of Response
- Oral Ulcers: Daily Average
- Overview
- ACR20 Response
- Oligoarticular PsA
- Joint Tenderness and Swelling
- Dactylitis
- Enthesitis
- Fatigue
- Pain
- Physical Function
- cDAPSA
- Overview
- Adverse Reactions Through Week 16
- Adverse Reactions Through 5 Years
- Tolerability
- FOREMOST Safety
- ACTIVE Safety
- Additional Safety
- Laboratory Parameters
No lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1 START TODAY WITHOUT DELAY
No lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1
START TODAY WITHOUT DELAY REFERENCESA small pill with a big history: 1 million+ patients treated globally since 2014 1-3,* PsO SAFETY PsA SAFETY
A small pill with a big history: 1 million+ patients treated globally since 2014 1-3,*
PsO SAFETY PsA SAFETY REFERENCES & FOOTNOTE
OTEZLA XR ONCE-DAILY IS AVAILABLE 1
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.