A small pill with a big history: 1 million+ patients treated globally since 2014 1-3,* PsO SAFETY PsA SAFETY
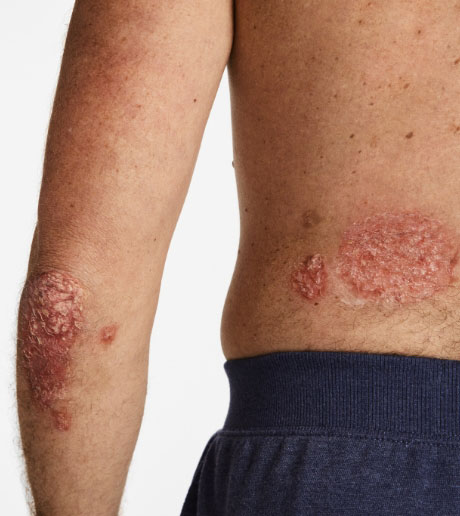
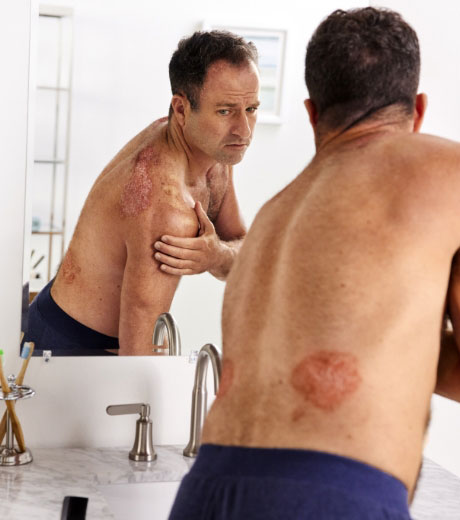
4 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
4 INDICATIONSOtezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.
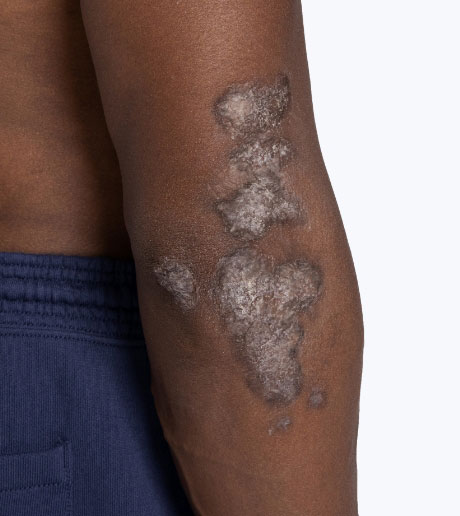
- Otezla is indicated for the treatment of pediatric patients 6 years of age and older and weighing at least 20 kg with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
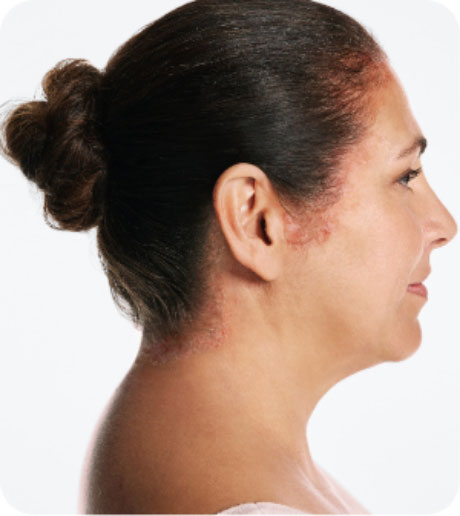
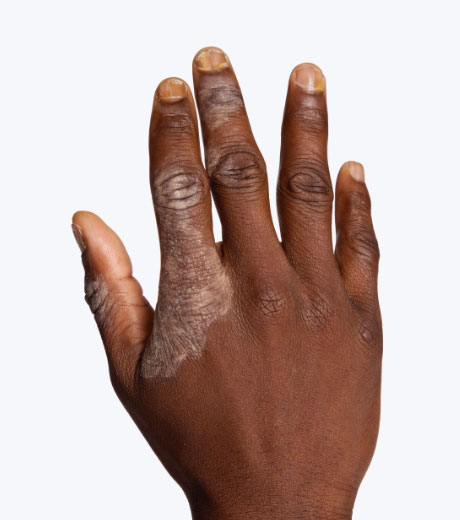
- Otezla is indicated for the treatment of adult patients with active psoriatic arthritis.
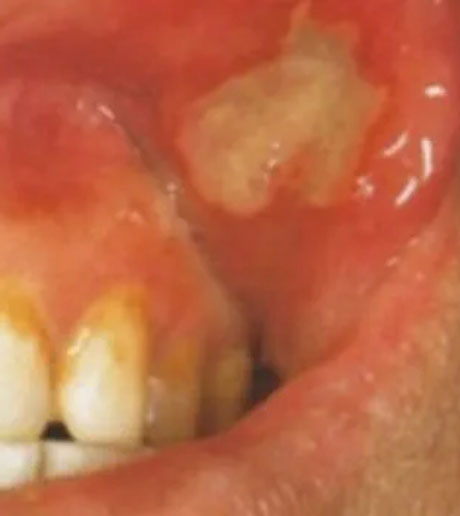
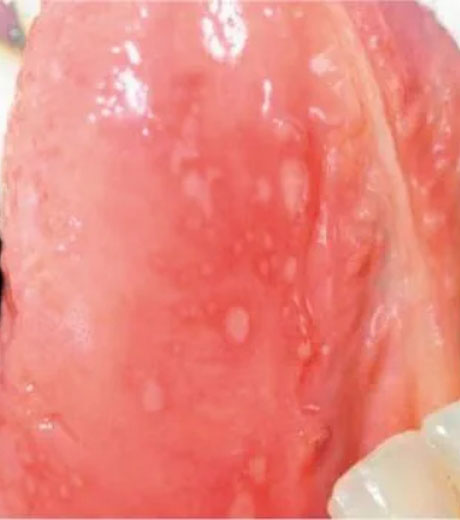
- Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
- Clinical Response of Oral Ulcers
- Oral Ulcers: Pain Results
- Oral Ulcers: Complete Response
- Oral Ulcers: Maintenance of Response
- Oral Ulcers: Daily Average
- Overview
- ACR20 Response
- Oligoarticular PsA
- Joint Tenderness and Swelling
- Dactylitis
- Enthesitis
- Fatigue
- Pain
- Physical Function
- cDAPSA
- Overview
- Adverse Reactions Through Week 16
- Adverse Reactions Through 5 Years
- FOREMOST Safety
- ACTIVE Safety
- Additional Safety
- Laboratory Parameters
- Overview
- Mild to Moderate
- PASI-75 Response
- Mean PASI Response
- Pediatric Response
- Scalp Response
- Nail Response
- Itch Response
- Genital Response
- Patient Photo Library
- Real-World Data
First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA
SEE THE DATA REFERENCESNo lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1 START TODAY WITHOUT DELAY
No lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1
START TODAY WITHOUT DELAY REFERENCESA small pill with a big history: 1 million+ patients treated globally since 2014 1-3,* PsO SAFETY PsA SAFETY
A small pill with a big history: 1 million+ patients treated globally since 2014 1-3,*
PsO SAFETY PsA SAFETY REFERENCES & FOOTNOTEFirst and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA
SEE THE DATA REFERENCESNo lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1 START TODAY WITHOUT DELAY
No lab monitoring. No TB or baseline blood panel tests. No planning around live vaccines 1
START TODAY WITHOUT DELAY REFERENCESA small pill with a big history: 1 million+ patients treated globally since 2014 1-3,* PsO SAFETY PsA SAFETY
A small pill with a big history: 1 million+ patients treated globally since 2014 1-3,*
PsO SAFETY PsA SAFETY REFERENCES & FOOTNOTE*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.