First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
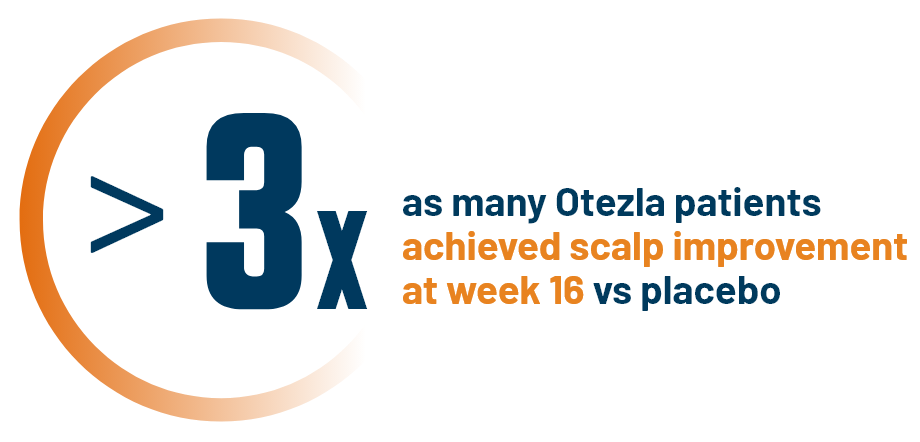
RESULTS SEEN IN OTEZLA PATIENTS
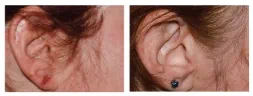
BASELINE
WEEK 16
ScPGA: 0
3-point improvement
in ScPGA score
3-point improvement
in ScPGA score
Actual clinical trial patient from STYLE. 4
Individual results may vary.
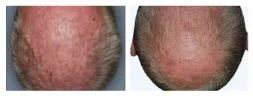
BASELINE
WEEK 16
ScPGA: 2
1-point improvement in ScPGA score
1-point improvement in ScPGA score
Actual clinical trial patient from STYLE. 4
Individual results may vary.
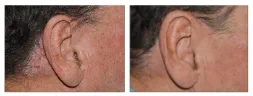
BASELINE
WEEK 16
ScPGA: 3
1-point improvement in ScPGA score
1-point improvement in ScPGA score
Actual clinical trial patient from STYLE. 4
Individual results may vary.