First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
PATIENT’S ASSESSMENT OF PAIN IN DMARD-NAÏVE PATIENTS THROUGH 5 YEARS 2
PALACE 4: Mean change in patient's assessment of pain VAS
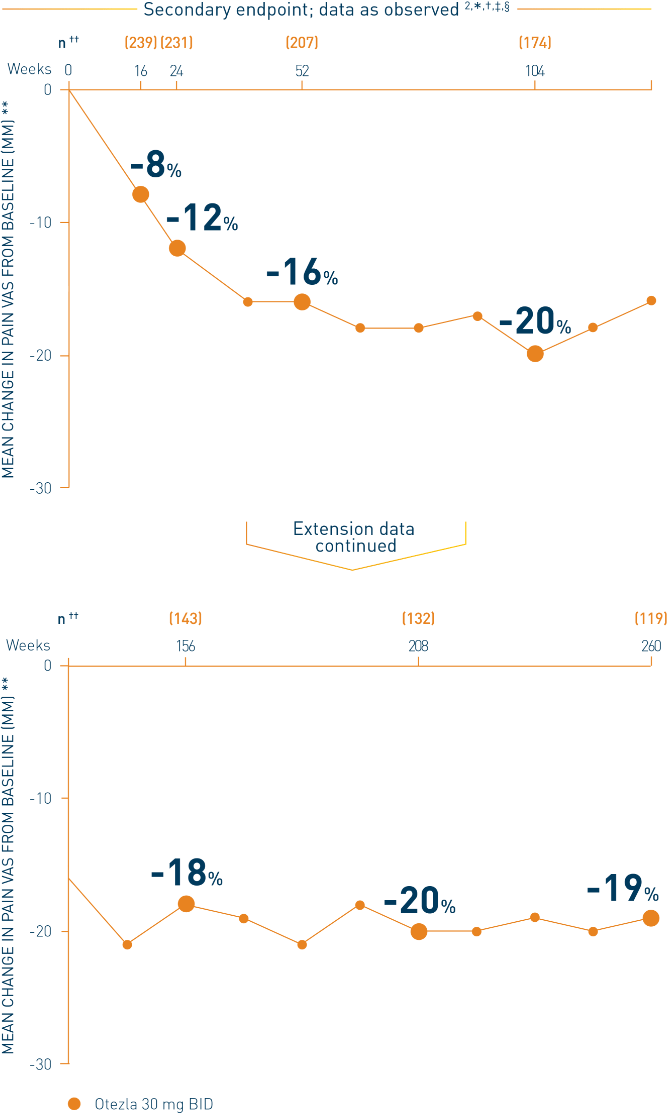
*Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. †Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡Patients discontinued treatment during the study due to adverse events, lack of efficacy, and other (withdrawal by patient, loss of follow-up, protocol violation, noncompliance, and other). §Pain was assessed on a 0-100 mm VAS. **Mean assessment of pain VAS score at baseline for patients receiving Otezla 30 mg BID. ††The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes subjects who discontinued early between the preceding timepoint and the specific timepoint; patients with a zero value at baseline are excluded from the summary of percent change from baseline.
- Consider open-label extension (OLE) phase study limitations when interpreting results. The OLE was not blinded, not controlled, and included inherent self-selection bias. Overall, from weeks 52 to 260, a total of 80 patients (31.5%) discontinued during the study, of which 16 patients (6.3%) discontinued due to adverse events 2,3,‡‡
‡‡The OLE period was from weeks 52 to 260.