First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
4 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
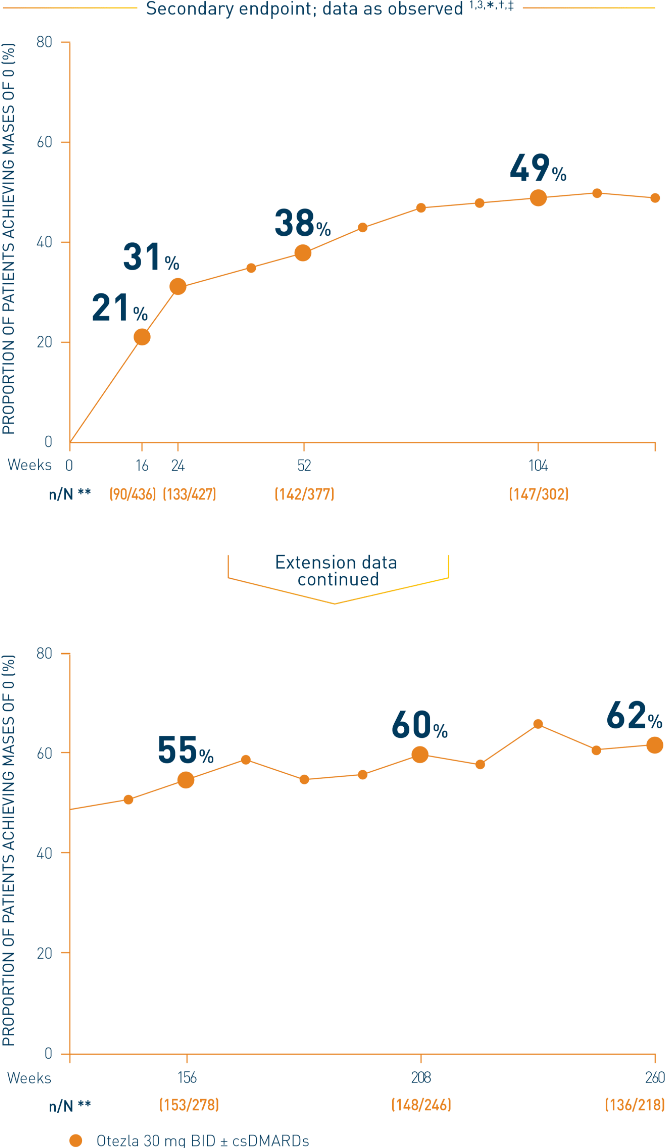
Mean MASES at baseline: Otezla 30 mg BID, 4.5; placebo, 4.6 3,††
*Patients with preexisting enthesitis. †Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. §MASES scale = 0-13; a score of 0 indicates complete resolution of enthesitis. **n/N number of responders/number of patients who had sufficient data for a definitive determination of response status at the timepoint, which includes patients who discontinued early between the preceding timepoint and the specific timepoint. ††Includes patients re-randomized to receive Otezla 30 mg BID at week 16 or week 24.
‡‡The OLE period was from weeks 52 to 260.
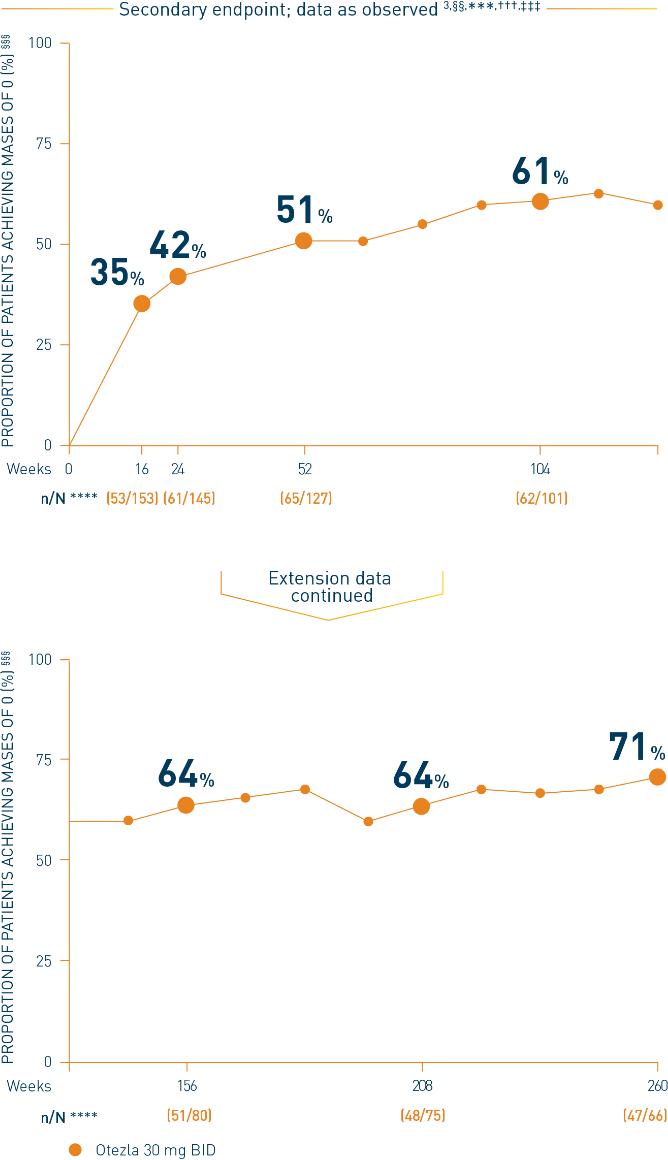
Mean MASES at baseline: Otezla 30 mg BID, 3.7; placebo, 3.6 3
§§Examined among patients with enthesitis at baseline (n=162). ***Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. †††Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡‡‡Patients discontinued treatment during the study due to adverse events, lack of efficacy, and other (withdrawal by patient, loss of follow-up, protocol violation, noncompliance, and other). §§§MASES scale = 0-13; a score of 0 indicates complete resolution of enthesitis. ****n/N, number of responders/number of subjects who had sufficient data for a definitive determination of response status at the timepoint, which includes subjects who discontinued early between the preceding timepoint and the specific timepoint.
††††The OLE period was from weeks 52 to 260.
BID, twice daily; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DMARD, disease-modifying antirheumatic drug; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; PsA, psoriatic arthritis.
Contraindications
Otezla® (apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
Otezla® is indicated for the treatment of:
References: 1. Kavanaugh A, Gladman DD, Edwards CJ, et al. Arthritis Res Ther. 2019;21(1):118. 2. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 3. Data on file, Amgen Inc. 4. Wells AF, Edwards CJ, Kivitz AJ, et al. Rheumatology (Oxford). 2018;57(7):1253-1263.