A Treat-to-Target Approach for Patients with Psoristic Arthritis Using cDAPSA, click here
Otezla Is an Oral Treatment Option for Use in Psoriatic Arthritis Patients 1
Otezla®, apremilast, is an oral treatment option for first-line systemic use in patients with psoriatic arthritis.
Please see Important Safety Information within this video and Full Prescribing Information on OtezlaPro.com.
Indication:
Otezla® (apremilast) is indicated for the treatment of adult patients with active psoriatic arthritis.
Contraindications: Otezla is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation.
Before we explore the clinical trials for Otezla in patients with psoriatic arthritis, 1 let’s look at some important safety information.
Warnings and Precautions:
Hypersensitivity:
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapy.
Diarrhea, Nausea, and Vomiting:
Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting.
Depression:
Carefully weigh the risks and benefits of treatment with Otezla for patients with a history of depression and/or suicidal thoughts or behavior, or in patients who develop such symptoms while on Otezla. Patients, caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts, or other mood changes, and they should contact their healthcare provider if such changes occur. Treatment with Otezla is associated with an increase in depression. During clinical trials, 1.0% reported depression or depressed mood compared to 0.8% treated with placebo. Suicidal ideation and behavior was observed in 0.2% of patients on Otezla, compared to none in placebo-treated patients. Depression was reported as serious in 0.2% of patients exposed to Otezla, compared to none in placebo-treated patients. Two patients who received placebo committed suicide compared to none on Otezla.
Weight Decrease:
Monitor body weight regularly; evaluate unexplained or clinically significant weight loss, and consider discontinuation of Otezla. Body weight loss of 5 to 10% was reported in 10% of patients taking Otezla and in 3.3% of patients taking placebo.
Drug Interactions:
Apremilast exposure was decreased when Otezla was co-administered with rifampin, a strong CYP450 enzyme inducer; loss of Otezla efficacy may occur. Concomitant use of Otezla with CYP450 enzyme inducers—for example, rifampin, phenobarbital, carbamazepine, phenytoin—is not recommended.
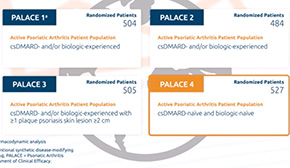
Otezla was evaluated in one of the largest global clinical development programs ever conducted in psoriatic arthritis. 1 The approval of Otezla was based on the results of 3 multicenter, randomized, placebo-controlled phase 3 studies: PALACE 1, 2, and 3. 2 The efficacy and safety of Otezla were examined in disease-modifying antirheumatic drug, or DMARD, and/or biologic-naïve patients in the PALACE 4 study. 3
Let’s focus our attention on PALACE 4 and Otezla in DMARD- and/or biologic-naïve patients. 3 All 4 PALACE studies enrolled patients of 18 or more years of age with active psoriatic arthritis, defined as having at least 3 swollen joints and at least 3 tender joints. 1-4 Patients in PALACE 1, 2, and 3 had active psoriatic arthritis, despite prior or current DMARD therapy. 1-3 Only DMARD-naïve patients were allowed in PALACE 4 and the only concomitant therapies allowed were NSAIDs and corticosteroids. 4
All 4 studies had a similar design. During the placebo-controlled phase, patients were randomized to receive Otezla 20 mg or
30 mg twice daily or placebo. 1,2 The primary endpoint was the percentage of patients achieving an ACR20 response at Week 16. 1,2 All patients in the placebo group whose tender or swollen joint counts had not improved by at least 20% at Week 16 were
re-randomized to one of the Otezla treatment groups. 1,2 At Week 24, patients entered the blinded active-treatment phase, and all remaining placebo patients were re-randomized to active treatment with Otezla. 1,2 After 1 year, patients could continue in the long-term open-label extension of the trial.
Despite being DMARD-naïve, PALACE 4 patients had considerable disease activity at baseline; the mean duration of disease at baseline was 3.4 years, and the mean disease activity score in 28 joints using C-reactive protein—or DAS28-CRP—was 4.6. 1,2 In PALACE 1-3, the mean age was 50 years, the mean duration of psoriatic arthritis at baseline was 7.4 years, and the mean
DAS28-CRP was 4.7. 2
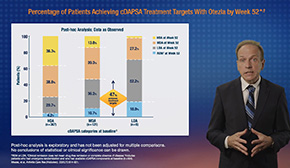
Significantly more patients on Otezla 30 mg twice daily met the primary endpoint of ACR20 response at Week 16 than patients on placebo in all 4 PALACE studies. In PALACE 4, 31% of DMARD-naïve patients on Otezla achieved an ACR20 response at
Week 16 compared with 16% of patients on placebo. 1-6 ACR20 response was captured through 5 years. 1 In PALACE 4, the proportion of patients achieving an ACR20 response was 66% at 5 years. 1 Consider open label extension study limitations when interpreting results. The open label extension is not blinded, not controlled, and includes inherent self-selection bias. 1
Of importance to patients with psoriatic arthritis, Otezla has 5-year data on the following manifestations and symptoms. Let’s dive deeper into these data. 1
Joint tenderness and swelling are 2 of the main symptoms of psoriatic arthritis. 1 At 5 years, the mean percent reduction in tender joint count, or TJC, was -76% and the mean percent reduction in swollen joint count, or SJC, was -85%. 1
Dactylitis and enthesitis are also distinguishing features of psoriatic arthritis. 1 Based on a review of 157 publications on dactylitis and enthesitis in patients with psoriatic arthritis, dactylitis occurs in up to 53% of patients and enthesitis occurs in up to 78% of patients with psoriatic arthritis. 2 Of the patients in PALACE 4 with preexisting dactylitis, the percentage of patients whose dactylitis resolved was 39% at Week 16 and 95% at 5 years. 1 In PALACE 4, of the DMARD-naïve patients with preexisting enthesitis, the percentage of patients who achieved resolution of enthesitis was 35% at Week 16 and 71% at 5 years. 1
Let’s turn our attention to the safety profile of Otezla.
Adverse Reactions:
The most common adverse reactions, 5% or more, are diarrhea, nausea, and headache.
Use in Specific Populations:
Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss.
The most common adverse reactions with Otezla were diarrhea, nausea, and headache.
The majority of diarrhea and nausea generally occurred within the first 2 weeks of treatment and tended to resolve
within 4 weeks with continued dosing. 1 There have been reports of severe cases of diarrhea, nausea, and vomiting associated with the use of Otezla. In some cases patients were hospitalized. Be sure to monitor patients who are more susceptible to complications of diarrhea or vomiting. 2 Overall through Week 24, 3.4% of patients taking Otezla discontinued treatment because of any adverse reaction compared with 2.3% of placebo-treated patients. 1
The long-term safety profile of Otezla was consistent through 5 years of exposure in the PALACE 4 clinical trial study of DMARD-naïve patients with active psoriatic arthritis. 1
Otezla is an oral treatment for adults with active psoriatic arthritis. It has the first and largest study in DMARD-naïve psoriatic arthritis patients and may be used as a first-line systemic treatment option. Furthermore, it has 5 years of efficacy and safety data available. 1,2 For more information about Otezla and its other indications, including the efficacy and safety profile, please see the full Prescribing Information for Otezla, provided on OtezlaPro.com.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen, Inc. 2. Wells AF, Edwards CJ, Kivitz AJ, et al. Rheumatology (Oxford). 2018;57:1253-1263. 3. Data on file, Amgen Inc. 4. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Ann Rheum Dis. 2014;73:1020-1026. 5. Cutolo M, Myerson GE, Fleischmann RM, et al. J Rheumatol. 2016;43:1724-1734. 6. Edwards CJ, Blanco FJ, Crowley J, et al. Ann Rheum Dis. 2016;75:1065-1073. 7. Gottlieb A, Korman NJ, Gordon KB, et al. J Am Acad Dermatol.2008;58:85-864. 8. Mease PJ, Armstrong AW. Drugs. 2014;74:423-441. 9. Sakkas LI, Alexiou I, Simopoulou T, et al. Semin Arthritis Rheum. 2013;43:325-334.
Otezla Is the First and Only FDA-Approved Therapy for Adult Patients with Oral Ulcers Associated with Behçet’s Disease
Otezla®, apremilast, is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
Please see Important Safety Information within this video and Full Prescribing Information on OtezlaPro.com.
Behçet’s Disease is a rare, chronic, and variable vasculitis that causes inflammation of the arteries and veins across multiple organ systems. 1-3 Hallmark characteristics of Behçet’s Disease include aphthous ulcers, commonly occurring in the oral mucosa. 1
The clinical manifestations of Behçet’s Disease are heterogeneous and can vary from patient to patient. 1 Despite these differences, the majority of patients with Behçet’s Disease frequently experience recurrent oral ulcers as their first manifestation. These can be bothersome and painful. 1-4
Other clinical manifestations of Behçet’s Disease include, in decreasing prevalence, genital ulcers, skin lesions, ocular involvement, joint involvement, vascular involvement, neurological involvement, and gastrointestinal involvement. 2,5,6 Understanding the many manifestations of Behçet’s Disease can help with diagnosis.
The International Study Group or ISG criteria can be used to diagnose Behçet’s Disease by the presence of recurrent oral ulceration plus at least 2 of the following: recurrent genital ulceration, eye lesions, skin lesions, or a positive result on pathergy testing. Otezla is the first and only therapy approved for adult patients with oral ulcers associated with Behçet’s Disease.
Indication:
Otezla® (apremilast) is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
Contraindications: Otezla is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation.
Warnings and Precautions:
Hypersensitivity:
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapy.
Diarrhea, Nausea, and Vomiting:
Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting.
Depression:
Carefully weigh the risks and benefits of treatment with Otezla for patients with a history of depression and/or suicidal thoughts/behavior, or in patients who develop such symptoms while on Otezla. Patients, caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and they should contact their healthcare provider if such changes occur. Treatment with Otezla is associated with an increase in depression. During the clinical trial, 1% (1/104) reported depression or depressed mood compared to 1% (1/103) treated with placebo. No instances of suicidal ideation or behavior were reported in patients treated with Otezla or treated with placebo.
Weight Decrease:
Monitor body weight regularly; evaluate unexplained or clinically significant weight loss, and consider discontinuation of Otezla. Body weight loss of more than 5% was reported in 4.9% of patients taking Otezla and in 3.9% of patients taking placebo.
Drug Interactions:
Apremilast exposure was decreased when Otezla was co-administered with rifampin, a strong CYP450 enzyme inducer; loss of Otezla efficacy may occur. Concomitant use of Otezla with CYP450 enzyme inducers (for example, rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended.
Otezla was evaluated in 207 adult patients with active oral ulcers associated with Behçet’s Disease in the phase 3, multicenter, randomized, placebo-controlled RELIEF study.
All patients in the study met the ISG criteria for Behçet’s Disease, had 1 or more prior treatments with non-biologic therapy, had at least 2 oral ulcers at screening and randomization, had at least 3 occurrences of oral ulcers within 12 months prior to randomization, and were candidates for systemic therapy. 1,2 Patients were excluded if they had active major organ involvement or prior biologic therapy for oral ulcers associated with Behçet’s Disease. 1,2
In this study, patients were randomized 1:1 to receive either Otezla 30 mg twice daily or placebo for the first 12 weeks of the placebo-controlled treatment phase. 1 After week 12, all patients received Otezla 30 mg twice daily for up to 64 weeks during the active treatment phase. 2
Of note, the mean age of patients included in the RELIEF study was 40 years, with a mean duration of Behçet’s Disease of
6.8 years. The mean baseline oral ulcer count was 4.2 for the Otezla arm and 3.9 for the placebo arm.
The measures of oral ulcers observed at week 12 included the following: change from baseline in the pain of oral ulcers as measured by visual analog scale scores at week 12; proportion of patients achieving oral ulcer complete response, meaning oral ulcer free, at week 12; proportion of patients achieving oral ulcer complete response by week 6, and who remained oral ulcer–free for at least 6 additional weeks during the 12-week placebo-controlled treatment phase; and daily average number of oral ulcers during the 12-week placebo-controlled treatment phase.
Let’s first look at the effect of Otezla on oral ulcer pain.
The change from baseline in oral ulcer pain, as measured by VAS scores, was statistically greater in patients treated with Otezla vs placebo, with a treatment difference of -24.1.
Now let’s look at the effect on oral ulcer complete response, characterized by the patients who were oral ulcer–free
at week 12.
There was a significantly greater proportion of patients achieving oral ulcer complete response at week 12 when treated with Otezla vs placebo, with a treatment difference of 31%.
Next, let’s look at maintenance of oral ulcer complete response.
30% of patients taking Otezla who achieved oral ulcer complete response by Week 6 remained oral ulcer–free for at least 6 additional weeks during the 12-week phase compared with 5% of patients treated with placebo, which accounts for a significant treatment difference of 25%.
Lastly, let’s look at the effect of Otezla on the number of oral ulcers over 12 weeks.
Patients taking Otezla experienced a significantly lower daily average number of oral ulcers compared with patients taking placebo.
Let’s turn our attention to the safety profile for Otezla.
Adverse Reactions: The most common adverse reactions, 10% or more, are diarrhea, nausea, headache, and upper respiratory tract infection.
The majority of patients reporting diarrhea, nausea, and vomiting did so within the first week of treatment, and the events tended to resolve over time. 1 Postmarketing reports of severe diarrhea, nausea, and vomiting have been associated with the use of Otezla. In some cases patients were hospitalized. It is important to monitor patients who are more susceptible to complications of diarrhea or vomiting. 2
During the placebo-controlled period of the study, 2.9% of patients taking Otezla and 4.9% of those taking placebo discontinued treatment due to adverse reactions. 1
Use in Specific Populations:
Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss.
Otezla is the first and only approved therapy indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease. For more information about Otezla and its other indications, including the efficacy and safety profile, please see the full Prescribing Information for Otezla, provided on OtezlaPro.com.
References: 1. Leonardo NM, McNeil J. Int J Rheumatol. 2015;2015:945262. 2. Zeidan MJ, Saadoun D, Garrido M, et al. Auto Immun Highlights. 2016;7. 3. Koné-Paut I. Pediatr Rheumatol Online J. 2016;14:10. 4. Kokturk A. Patholog Res Int. 2012;2012:690390. 5. Alpsoy E, Donmez L, Onder M, et al. Br J Dermatol. 2007;157(5):901-906. 6. Alpsoy E. J Dermatol. 2016;43(6):620-632. 7. Melikoğlu M, Melikoğlu MA. Acta Reumatol Port. 2014;39(1):46-53. 8. Barnes CG. History and diagnosis. In: Behçet’s Syndrome. 2010. 9. Verity DH, Wallace GR, Vaughan RW, et al. Br J Ophthalmol. 2003;87(9):1175-1183. 10. Otezla [package insert]. Thousand Oaks, CA: Amgen, Inc. 11. Data on file, Amgen Inc.
Mechanism of Action in Psoriatic Arthritis, click here