First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
4 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
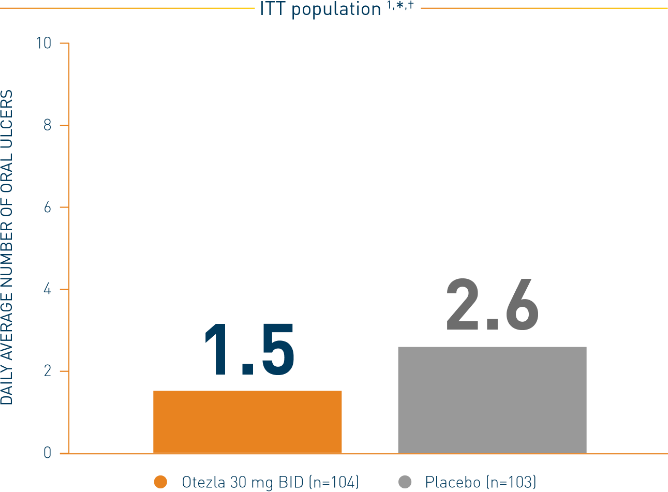
Statistically significant treatment difference ‡: (95% CI) = -1.1 (-1.6, -0.7)
*Mean daily averages are least-squares means from analysis of covariance, after adjusting for sex, region, and baseline number of oral ulcers. †Based on oral ulcer counts measured at baseline and at weeks 1, 2, 4, 6, 8, 10, and 12. ‡Otezla vs placebo.
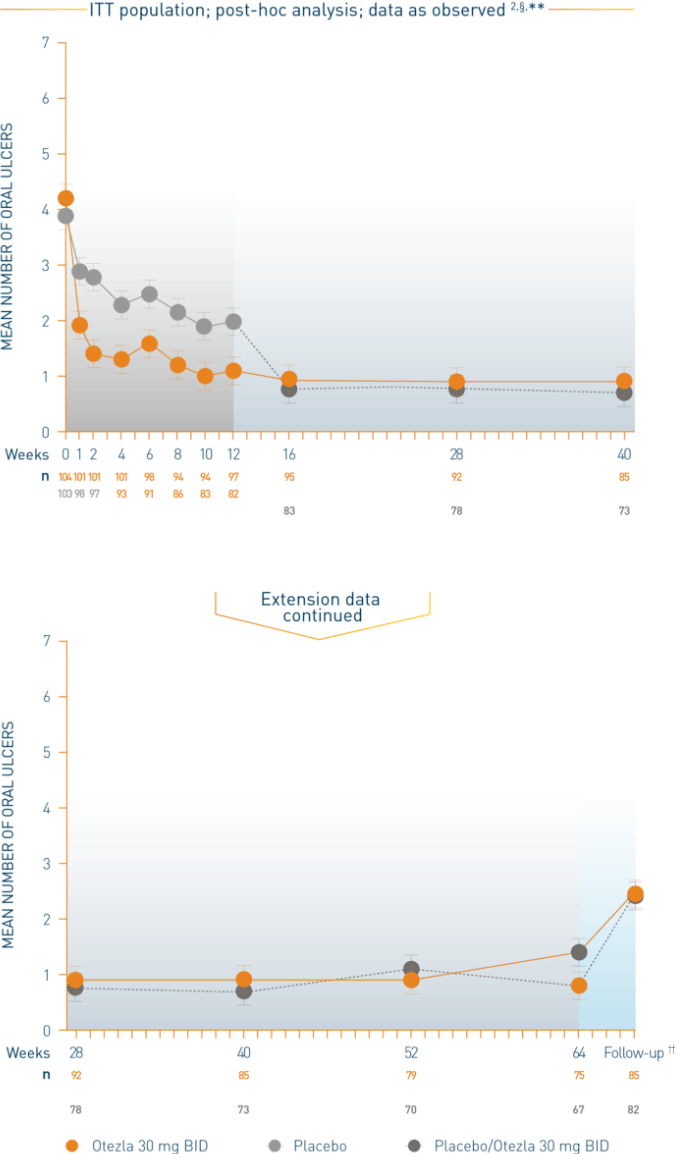
Patients initiated on placebo were switched to Otezla 30 mg BID at week 12
All patients in both arms received Otezla 30 mg BID at week 12 through week 64
Patients entered a 4-week, post-treatment observational follow-up period following discontinuation of Otezla at or before week 64
§Post-hoc analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn. **Data are presented “as observed” with no imputation for missing values. ††Patients entered a 4-week, post-treatment, observational follow-up phase following discontinuation of Otezla at or before week 64.
BID, twice daily; CI, confidence interval; ITT, intent to treat; SE, standard error.
Contraindications
Otezla® (apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
Otezla® is indicated for the treatment of:
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Hatemi G, Mahr A, Ishigatsubo Y, et al. N Engl J Med. 2019;381(20):1918-1928.