First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
OTEZLA:
4 INDICATIONSOtezla® (apremilast)/Otezla XR™ (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
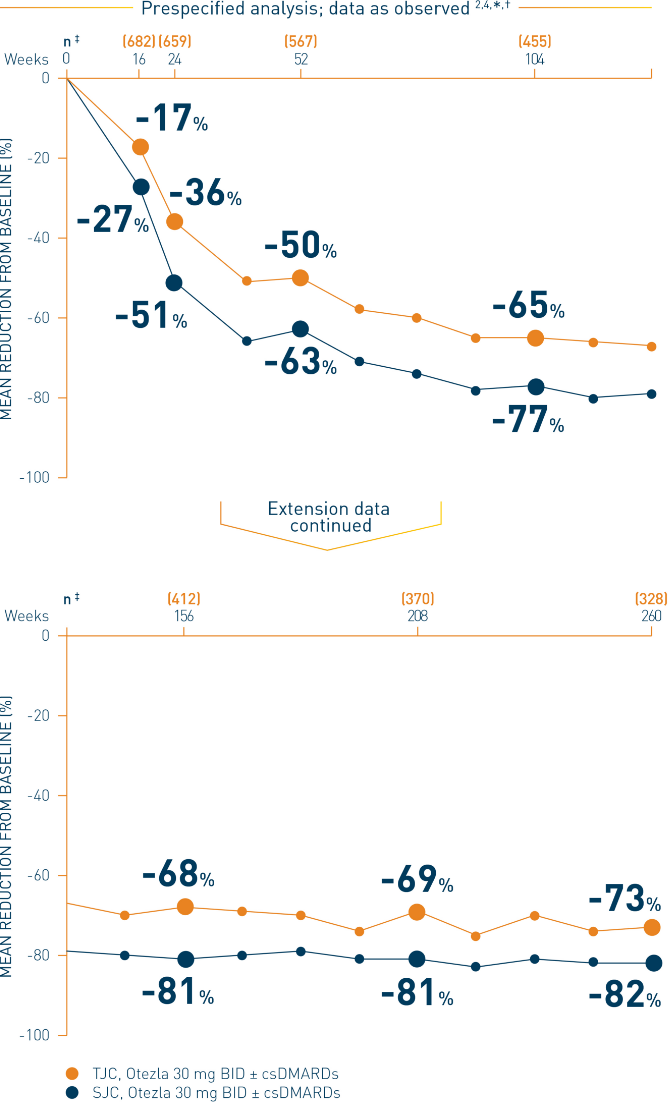
*Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. †Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes patients who discontinued early between the preceding timepoint and the specific timepoint.
§The OLE period was from weeks 52 to 260.
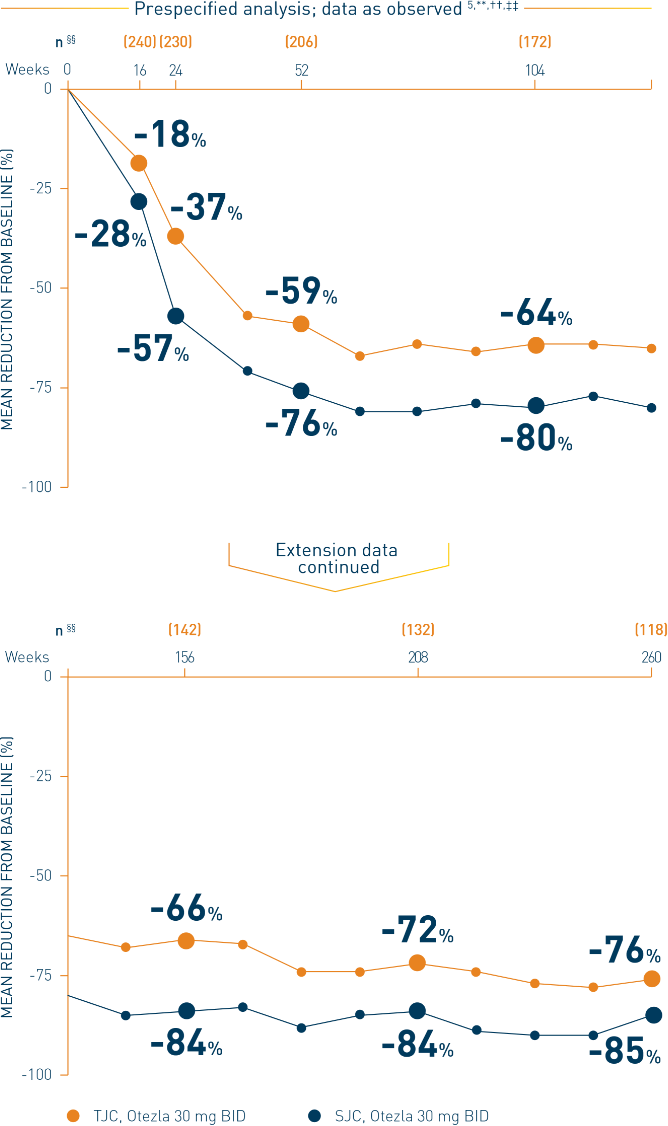
**Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. ††Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡‡Patients discontinued treatment during the study due to adverse events, lack of efficacy, and other (withdrawal by patient, loss of follow-up, protocol violation, noncompliance, and other). §§The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes subjects who discontinued early between the preceding timepoint and the specific timepoint.
***The OLE period was from weeks 52 to 260.

†††Patients with ≤4 active joints at baseline. Based on all joints. One active joint could be swollen, tender, or both.
Post-hoc analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
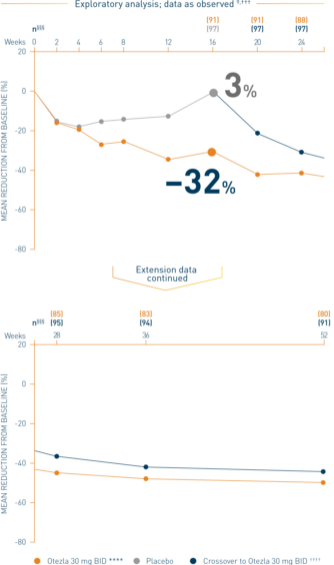
‡‡‡Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 52 weeks. §§§The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes subjects who discontinued early between the preceding timepoint and the specific timepoint. ****Includes all patients exposed to Otezla from baseline who completed treatment through week 52. ††††At week 16, patients receiving placebo were eligible to be switched to Otezla; at week 24, all remaining patients receiving placebo were switched to Otezla. Only patients who received ≥1 dose of Otezla were included.
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn. 2
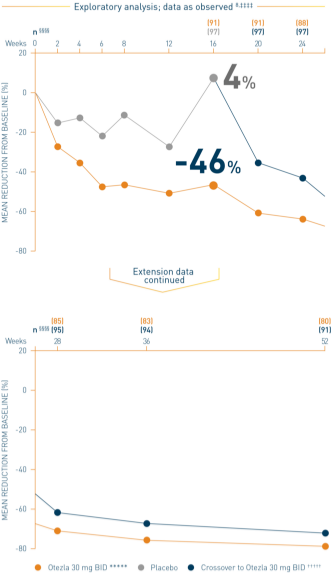
‡‡‡‡Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 52 weeks. §§§§The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes subjects who discontinued early between the preceding timepoint and the specific timepoint. *****Includes all patients exposed to Otezla from baseline who completed treatment through week 52. †††††At week 16, patients receiving placebo were eligible to be switched to Otezla; at week 24, all remaining patients receiving placebo were switched to Otezla. Only patients who received ≥1 dose of Otezla were included.
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn. 2
BID, twice daily; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DMARD, disease-modifying antirheumatic drug; PsA, psoriatic arthritis; SJC, swollen joint count; TJC, tender joint count.
Contraindications
Otezla/OTEZLA XR is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla/OTEZLA XR and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
References: 1. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. J Rheumatol. 2015;42(3):479-488. 2. Data on file, Amgen Inc. 3. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 4. Kavanaugh A, Gladman DD, Edwards CJ, et al. Arthritis Res Ther. 2019;21(1):118. 5. Wells AF, Edwards CJ, Kivitz AJ, et al. Rheumatology (Oxford). 2018;57(7):1253-1263. 6. Mease P, Gladman D, Coates LC, et al. 16-week results from FOREMOST, a placebo-controlled study involving oligoarticular psoriatic arthritis treated with apremilast. Presented at: ACR/ARHP Annual Meeting; November 10-15, 2023; San Diego, CA. 7. Gossec L, Gladman D, Coates L, et al. Early oligoarticular psoriatic arthritis responds to treatment with apremilast: week 16 results from FOREMOST—a phase 4 randomized controlled trial. Presented at: 32nd Annual Meeting of the European Academy of Dermatology and Venereology (EADV); October 11-14, 2023; Berlin, Germany. 8. Mease P, Gladman D, Coates LC, et al. 16-week results from FOREMOST, a placebo-controlled study involving oligoarticular psoriatic arthritis treated with apremilast. Abstract 1691. ACR 2023, November 13, 2023. 9. Nash P, Ohson K, Walsh J, et al; ACTIVE Investigators. Ann Rheum Dis. 2018;77(5):690-698.