First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
SIGNIFICANTLY MORE PATIENTS ACHIEVED COMPLETE RESPONSE BY WEEK 6 AND REMAINED ORAL ULCER–FREE FOR AT LEAST 6 ADDITIONAL WEEKS DURING THE 12-WEEK PHASE 1
RELIEF: Proportion of patients achieving complete response of oral ulcers by
week 6 and remaining oral ulcer-free for at least 6 additional weeks
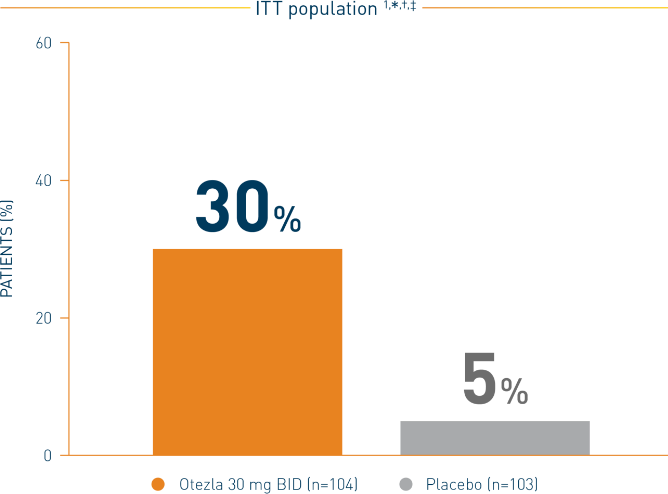
Statistically significant treatment difference §,**: 25% (95% CI) = 25% (16%, 35%)
*During the 12-week placebo-controlled treatment phase. †Patients for whom data are not available to determine response status are considered nonresponders. ‡Complete response is defined as the proportion of patients who are oral ulcer–free. §Adjusted difference in proportions is the weighted average of the treatment differences across the 4 strata of combined sex and region factors with the Cochran-Mantel-Haenszel weights. **Otezla vs placebo.




