First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
MEAN CHANGE IN FATIGUE SCORE THROUGH 5 YEARS 2
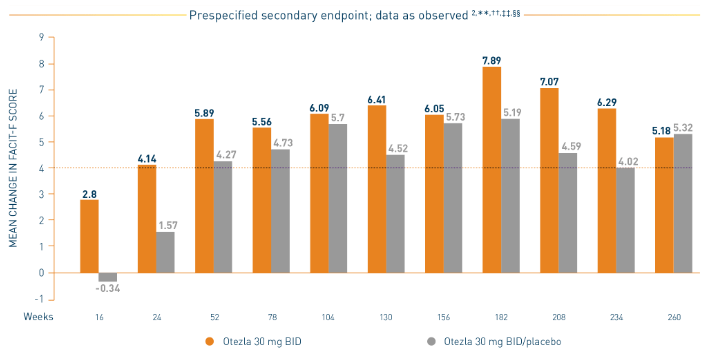
PALACE 1-3: Mean Change in FACIT-F
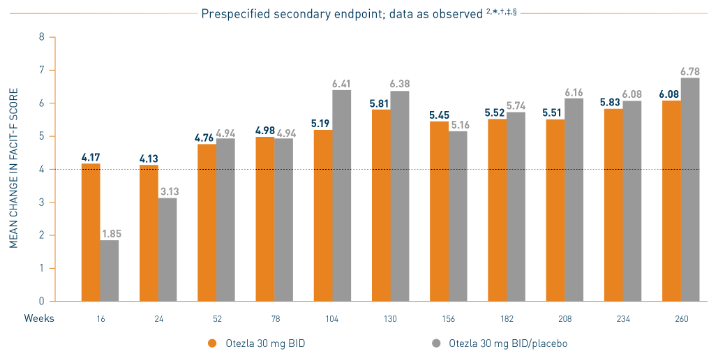
*Prespecified pooled analysis; weeks 16, 24, and 52 were prespecified secondary endpoints. †Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. ‡FACIT-F is a 13-item, self-administered questionnaire that assesses fatigue and its physical impact on daily activities and function. The total FACIT-F score ranges from 0 to 52, with lower scores denoting higher levels of fatigue.
- Consider open-label extension (OLE) phase study limitations when interpreting results. The OLE is not blinded, not controlled, and includes inherent self-selection bias. Overall, from weeks 52 to 260, a total of 156 patients (30%) discontinued during the study, of which 33 patients (6.3%) discontinued due to adverse events. 3,§
§The OLE period was from weeks 52 to 260.