First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
AT WEEK 12, OTEZLA IMPROVED MEASURES OF ORAL ULCERS 1
RELIEF: Clinical response of oral ulcers at week 12
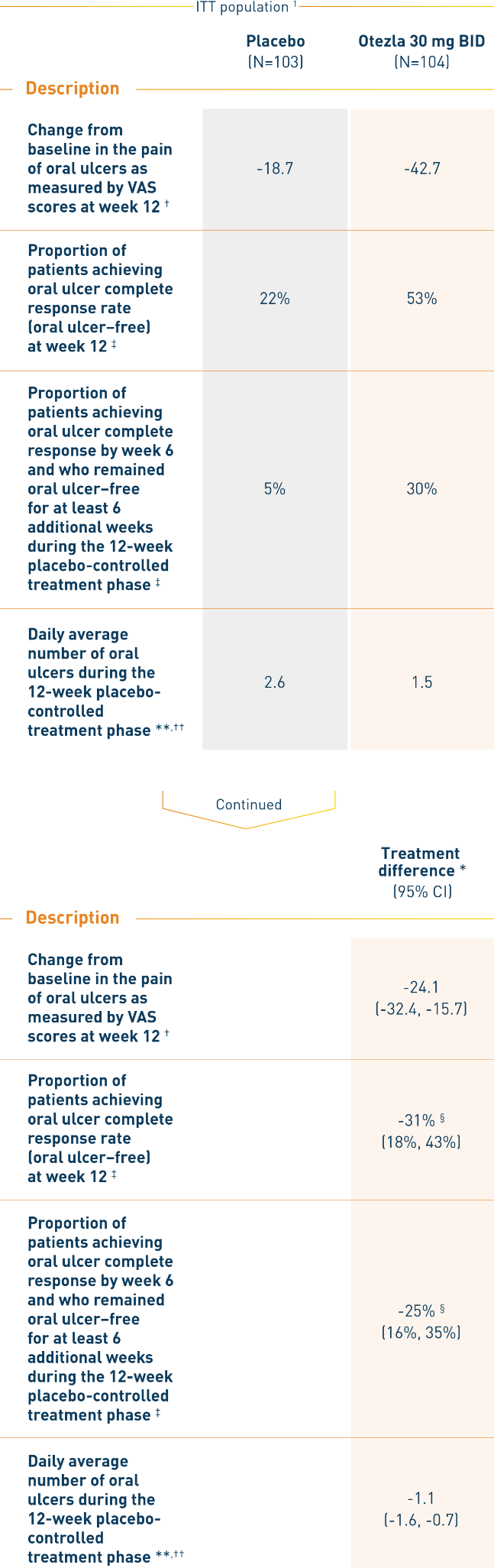
*Otezla—placebo. †Mean changes from baseline are least-squares means from mixed-effects model for repeated measures, adjusting for sex, region, and baseline pain of oral ulcers as measured by VAS: 0 = no pain, 100 = worst possible pain. ‡Patients for whom data are not available to determine response status are considered nonresponders. §Adjusted difference in proportions is the weighted average of the treatment differences across the 4 strata of combined sex and region factors with the Cochran-Mantel-Haenszel weights. **Mean daily averages are least-squares means from analysis of covariance, after adjusting for sex, region, and baseline number of oral ulcers. ††Based on oral ulcer counts measured at baseline and at weeks 1, 2, 4, 6, 8, 10, and 12.





