Mechanism of Action in Plaque Psoriasis
INDICATION
Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.
IMPORTANT SAFETY INFORMATION
Contraindications
Otezla® (apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation.
Please see additional Safety Information later in this video.
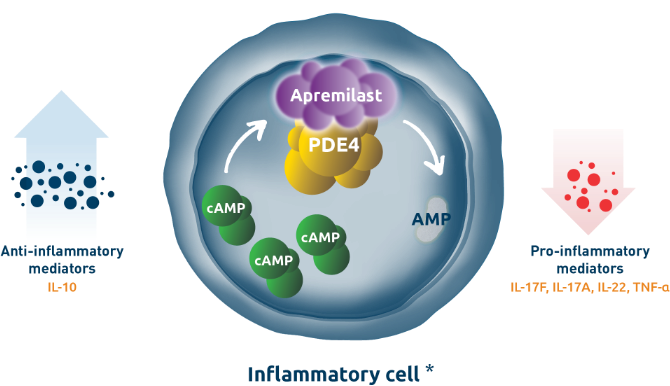
Plaque psoriasis appears on the skin as raised, red patches covered with a silvery buildup of dead skin cells. Plaque psoriasis most commonly appears on the knees, fingernails, elbows, lower back, and scalp. Data from cell and animal studies indicates that the immune cell releases pro-inflammatory cytokines that can then activate other cells. The activated cells recruit more cells to the site of the disease creating a chronic cycle of inflammation. Recent research has identified important signaling molecules within immune cells. One such molecule is phosphodiesterase 4 or PDE4, an intracellular enzyme that degrades cyclic AMP into its inactive form, AMP in inflammatory cells. A treatment for plaque psoriasis, apremilast, an inhibitor of PDE4 is indicated for the treatment of adult patients with plaque psoriasis. Apremilast is an oral small-molecule inhibitor of PDE4. The specific mechanisms by which apremilast exerts its therapeutic action is not well defined. Through this targeted inhibition, apremilast elevates intracellular cAMP levels, which is thought to decrease levels of some pro-inflammatory mediators and increase the production of certain anti-inflammatory mediators.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapy.
Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting.
Depression: Carefully weigh the risks and benefits of treatment with Otezla for patients with a history of depression and/or suicidal thoughts/behavior, or in patients who develop such symptoms while on Otezla. Patients, caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and they should contact their healthcare provider if such changes occur.
Treatment with Otezla is associated with an increase in depression. During clinical trials in patients with moderate to severe plaque psoriasis, 1.3% (12/920) of patients reported depression compared to 0.4% (2/506) on placebo. Depression was reported as serious in 0.1% (1/1308) of patients exposed to Otezla, compared to none in placebo-treated patients (0/506). Suicidal behavior was observed in 0.1% (1/1308) of patients on Otezla, compared to 0.2% (1/506) on placebo. One patient treated with Otezla attempted suicide; one patient on placebo committed suicide.
Weight Decrease: Monitor body weight regularly; evaluate unexplained or clinically significant weight loss and consider discontinuation of Otezla. Body weight loss of 5-10% occurred in 12% (96/784) of patients with moderate to severe plaque psoriasis treated with Otezla and in 5% (19/382) of patients treated with placebo. Body weight loss of ≥10% occurred in 2% (16/784) of patients treated with Otezla compared to 1% (3/382) of patients treated with placebo.
Drug Interactions: Apremilast exposure was decreased when Otezla was co-administered with rifampin, a strong CYP450 enzyme inducer; loss of Otezla efficacy may occur. Concomitant use of Otezla with CYP450 enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended.
Adverse Reactions
The most common adverse reactions (≥5%) are diarrhea, nausea, upper respiratory tract infection, and headache, including tension headache. Overall, the safety profile of Otezla in patients with mild to moderate plaque psoriasis was consistent with the safety profile previously established in adult patients with moderate to severe plaque psoriasis.
Use in Specific Populations
Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss.
Please see the Full Prescribing Information for Otezla provided on Otezlapro.com