First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
Study Design 1-3
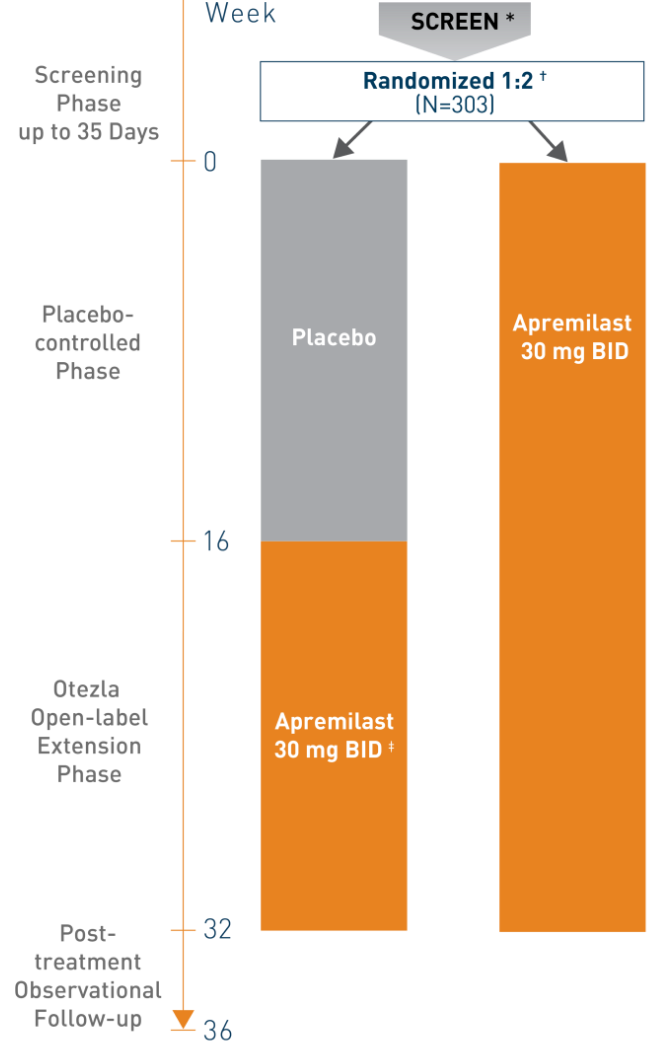
*Screening up to 35 days before randomization. †All doses were titrated over the first week of treatment. ‡At week 16, all placebo patients were switched to open-label apremilast 30 mg BID (with dose titration) through week 32.
-
Patients were randomized 2:1 to receive either Otezla (n=201) 30 mg BID or placebo (n=102) BID for week 16 (placebo-controlled phase) 2,3
- Randomization was stratified by baseline ScPGA score (3 [moderate] or 4 [severe]) to ensure balance between treatment arms
- At week 16, patients in the placebo arm were switched to Otezla 30 mg BID; patients initiated on Otezla continued with Otezla 30 mg BID until week 32 (weeks 16 to 32 open-label extension phase)




