A small pill with a big history: 1 million+ patients treated globally since 2014 1-3,* PsO SAFETY PsA SAFETY
Study Design 1
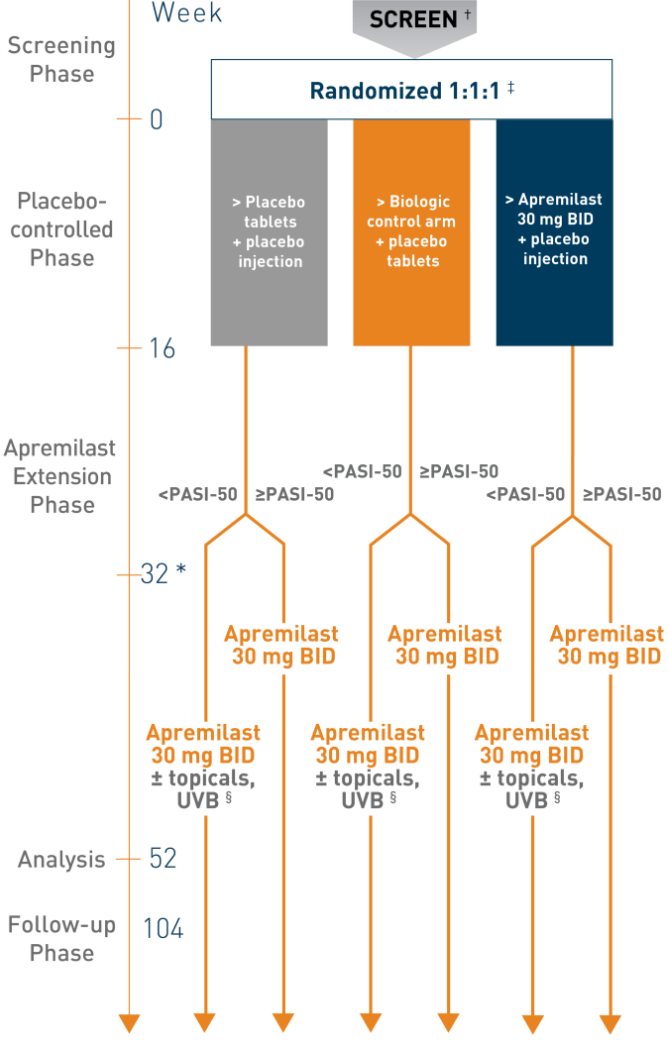
*Starting at week 32, all nonresponders (<PASI-50) had the option of adding topical therapies and/or ultraviolet light B phototherapy to their treatment regimen. †Screening up to 35 days before randomization. ‡For patients randomized to apremilast, doses were titrated over the first week of treatment. §At week 16, all placebo and biologic control arm patients were switched to open-label apremilast 30 mg BID through week 104.
- Patients were randomized 1:1:1 to either Otezla 30 mg (n=83) BID, biologic control arm (n=83) once weekly, or placebo (n=84) through week 16. At week 16, all patients crossed over to the Otezla arm 1
- This study was not designed for apremilast vs biologic control arm comparisons 1



